Reactive dye is the most important dye class for cellulosic materials. In the mid-1980s, worldwide consumption of reactive dye for cellulosic material was 10-12% whereas about 40% of total dye consumption was represented by Japan alone. It offers a wide range of dyes with varying shades, fastness with high brilliancy, and reproducibility. This article is all about the Overview and the Definition of Reactive Dye.
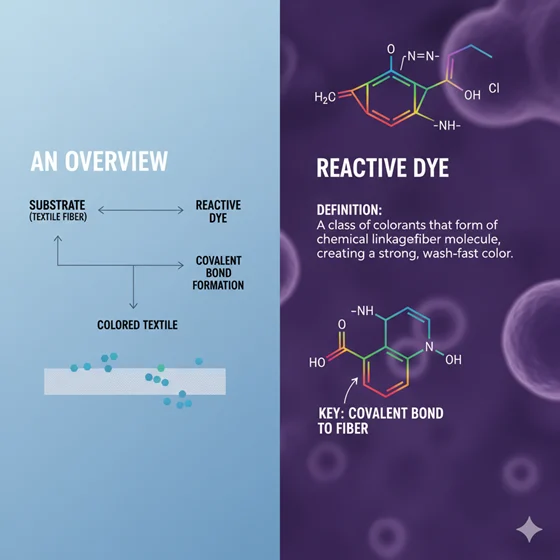
Looking for the reactive dye definition? You are on the right track! This article is all about reactive dye definition, along with its components, influencing factors, and, most importantly, dye hydrolysis. Reactive dye consists of a reactive group that makes covalent bonds with the fiber structure. Thus, it becomes a distinct part of the fiber. They are highly used for their varying shade range, fastness, and brilliancy. But 50% of the reactive dyeing cost is attributed to the washing off stages to remove hydrolysed dyes and the treatment for the resulting effluent.
Nowadays, in our country, reactive dye is extensively used to dye cellulosic materials. Honestly, when you visit most of the dyeing factories, you will find only reactive dyes there for cellulose dyeing purposes.
Reactive Dye Definition
Reactive dye consists of a chromophore and a reactive group, along with a solubilizing and bridging group. This is the only dye class that has a reactive group in its structure and forms a covalent bond with the polymeric system of the fiber.
Reactive dye + fiber = Reactive dye-fiber (by covalent bond)
Reactive dye is a dye class that has a reactive group in it, and it works like magic. It creates a covalent bond with the fiber and becomes an integral part of the fiber structure.
Good fastness properties, dye reproducibility, lower price, ease of dyeing method – all these features increase the popularity of dye day by day. Procion, Cibacron (Ciba), Remazol (Dystar) etc, are the most common dyes used by the factory.
History of Reactive Dye
In 1955, two chemists named Rattee and Stephen, who were working for ICI in England tried to invent a noble process for cotton dyeing. They used water-soluble dyes that contain dichlorotriazine groups. They applied the dye to the cellulose from a neutral bath and then increased the pH value.
They found that under mild alkaline conditions, a reactive chlorine atom on the triazine ring is substituted by an oxygen atom from the hydroxyl group of cellulose.
They succeeded and developed a new dye named REACTIVE DYE. An example of such a dye is C.I. Reactive Orange 1. Reactive dyes are now widely used for dyeing cellulosic fabrics. But protein fibers can also be dyed with reactive dyes.
Reactive dye components
Consider a general structure –
D-S-R-B
Where, D = Dye chromophore (Azo, Anthaquinone, Metal complex, Phthalocyanine group)
S = Solubilising group (-SO3Na)
R = Reactive group (-Cl, -Br, -SH, -OCH)
B = Bridging group (Amino, Oxide, Sulphur, Ethyl, Methyl group)
Influencing factors
- Temperature: Depends on the brand of dye
- pH: strong alkaline pH 10.5-11
- Time: 60 to 90 min
- Liquor ratio: A Higher liquor ratio is better
- Concentration of electrolyte: Determines the depth of shade
Reactive Dye Reaction
Typical reaction with cellulose:
Here, Cell-OH is cellulose, which has a reactive hydroxyl group
Dye-Cl is the dye that has a reactive chlorine group
Cell-O-Dye is dyed cellulose where the dye is linked to the cellulose by a covalent bond
D-SO2-CH2-CH2-OSO3Na + OH-Cell = D-SO2-CH2-CH2-O-Cell + NaHSO3
Reaction with protein fiber:
D-SO2-CH2-CH2-OSO3Na + NH2-Wool = D-SO2-CH2-CH2-NH-Wool + NaHSO3
D = Dye
Wool = Wool polymer
Cell = Cellulose polymer
Properties of Reactive Dye
- anionic in nature
- water-soluble dye
- found in powder, liquid, or paste form
- create a covalent bond with the textile fibers
- A certain amount of dye is hydrolyzed during dyeing ( 15-40% )
- They have good light fastness ( rating 6 )
- They have excellent wash fastness ( rating 4-5 ) as they attach to the fibers with a covalent bond ( a strong chemical process )
- They have moderate rubbing fastness
Classification
- Depending on the reactive group:
- Halogen (commonly chlorine) derivatives of nitrogen-containing heterocycle
- Activated vinyl compounds
Depending on the chemical constitution
Chloro triazine Reactive dyes.
- Monochloro dyes.
- Dichloro/Bifunctional dyes
- Trichloro dyes
- Depending on Reactivity
Low Reactive dye
Medium reactive dye
Highly reactive dye
- Depending on Temperature
Cold brand dyes
Hot brand dyes
Reactive dye – Why called so?
- Reactive dyes comprise a chromophore and a reactive group. In this way, they differ from other dyestuffs.
- They chemically react with the textile fibers by forming a covalent bond. They create a covalent bond with the terminal reactive hydroxyl group (-OH) of cellulose and the -NH2 group of protein fibers.
- The formation of covalent bond is a chemical process which is stronger than other bonds like Van der waals forces, hydrophobic bond, hydrogen bond or coulombic attraction of ions.
- Chemically cellulose behaves like polyhydric alchohol. Reactive dyes only react with cellulose under alkaline condition.
For this reason this dyes are called reactive dyes.
Reactive Dye Hydrolysis
Reactive dye is hydrolyzed and this is the most important drawbacks of it. You can’t keep your dye solution for long time once it is prepared. The reason is the high percentage of dye hydrolysis. About 20-40% reactive dye is hydrolyzed.
The reactive group of reactive dye reacts with the water instead of cellulose. It is called hydrolysis.
Mechanism of Reactive Dye
3 steps of dyeing mechanism of reactive dye.
- In exhaustion stage, dyeing starts from neutral bath. For substantivity some dyes are taken up by the fiber. In this stage, salt is added to promote dye uptake. Temperature also gradually increase.
- In fixation stage, alkali is added to increase the pH of the bath and this stage helps to fix the nucleophilic cellulosate ions with dyes.
- The rinsing process washes off and removes all the unfixed dye particles.
How to Use a Reactive Dye?
Wanna dye your favorite dress at home? Not to worry. Reactive dye would be a better solution.
Fiber Reactive Tie Dye
Tie dye is as simple as its name implies. You can easily do it at home. Color your favorite textiles with the simple ways.
The technique described below pretty much consists in preparing a dye bath in which the fabric is to be fully submerged.
Preparing dye bath
- Firstly, start by picking up your color.
- Your supplier will tell you about the amount you need to be used. But it is a wiser way to start with little and then take a look of it and if you think that you need a stronger color, then add more color to it.
- Using a whisker mix the color well in water.
Preparing the dye activator
- For the activator use washing soda or soda ash. For fixation of reactive dye we need alkali. So, here soda ash or washing soda should use for efficient dyeing.
- For a pound of fabric, use a gallon of water with five teaspoons of washing soda.
- Washing soda really likes to dissolve in hot water. So make sure your water is hot and pre-dissolve the washing soda into it so that you can use this later with dye solution.
- Use a whisker to completely dissolve the soda in water.
- At the very beginning, it may look cloudy but will become transparent soon as it dissolved completely.
Preparing Reactive Dye Fabric for Dyeing
- Soak the fabric in water once you tied and bundled it in your tied pattern. You can use rubber band for making knot.
- Before trying any dyeing technique, just wet the fabric well. Because it helps to penetrate the dye molecules inside the fabric.
- And yeah, this helps to get you the better result.
Dyeing steps
- Add all the ingredients in a single bath. Firstly the dye solution followed by the dye activator.
- Make sure to keep stirring by whisker to mix all of them very well.
- Now put the wet fabric and make sure it is submerged well.
- Use any spatula or use the whisker or your hand to keep the fabric underneath the water as it tends to float.
- Now you are done with it. Wait for an hour. In this time, dye-fiber reaction will take place.
- After an hour, remove the fabric from the bath and squeeze it and rinse well.
- After that dry and iron the fabric.
- Now it is perfectly ready to wear.
Reactive dye is a popular dye in the dyeing industry. Most of the cases the cellulose materials are dyed with reactive dye. The color brilliancy and easy usage along with the low price has made this dye so popular.
Hydrolysis of Reactive Dye
When alkaline condition is provided with reactive dye, they easily reacts with the hydroxyl group of cellulose. But if this situation is carried out for too long period, the dye molecules react with the hydroxyl group of water.
This results in concentration drop. Hydrolysis is the reaction that occurs between the dye particle and the hydroxyl group of water. 20-40% dye hydrolysis takes place in case of reactive dye.
- In case of Triazine dyes
- In case of Vinyl Sulphone dyes
Dye hydrolysis increases with the increasing following parameters:
- pH
- Dyeing temperature
- Time
- Amount of electrolyte
- No of reactive group present in the dye structure
- Reactivity of the dyes
- M:L ratio
How to reduce the dye hydrolysis?
Hydrolysis depends upon the reactivity of reactive groups as well as the no of reactive groups present in the dye structure. For each class of reactive dyes, a different application procedure is recommended.
Different classes of dyes require different temperatures for dyeing. The amount of alkali, temperature during dyeing, pH, as well as dyeing time should be kept minimum or as low as possible.
This can help in reducing dye hydrolysis. The contact time between dye and alkali is necessary for activation in the case of a reactive dye.
But this contact time should be kept as minimal as possible if you really want to reduce the hydrolysis of the reactive dye.
To Wrap Up!
Reactive dyes are not fast to chlorine bleach, unlike Vat dyes. Reactive dyed treads can’t be post-bleached. Again, the cost for the washing off process and the effluent treatment process is high for reactive dye.
- You may love to read: Basic Anatomy of Reactive dye
- Reactive Dye Printing Process: Experience Amazing Prints
- Difference Between Reactive and Vat Dyes


