Reactive dye is one of the most used dyes in Textiles, comparatively cheaper than many other dyes. Around 50% of total annual dyes consumption is reactive dyes. According to consumption Direct and Vat, dye holds 2nd and 3rd position respectively. But why reactive dyes are so popular? Definitely, it has some specific reasons. Let’s talk about this.
Reactive dyes is a cationic dyes. Cationic dyes this term express that the reactive portion of the dye holds a positive charge while reacting with the substrate.
Now the question arises what is the reactive portion of dye? Yes, it is the terminal group by which this chemical substrate (Dye) is attached to the fabric by a covalent bond and makes the applied substrate colorful. And for this reactive terminal group, this class of dyes is called reactive dyes.
In reactive dye, chromophore groups connect with the reactive group through Bridging groups. In reactive dyes structure chromophores are responsible for color. These dyes also contain other two groups the bridging group and the soluble group. Through Bridging groups chromophore groups are with reactive terminals. As dyeing takes place in the bath so dyes must be easily soluble in water for dyeing. And the responsibility of solubilizing groups is mentioned above which creates a covalent bond with fibers.
So, if consider a sketch then it will be..
C-B-S-R
Here C is the chromophore
B – Bridging Group
S- Solubilizing Group
R- Reactive Group
Chromophores: These are the chemical compounds responsible for product hue. They are color-bearing groups, that mainly contain complex compounds or legends. By the effect of chromogen depth or brightness of hue can be changed from dull to deep. Example: In figure (-N=N-) is the chromophore.
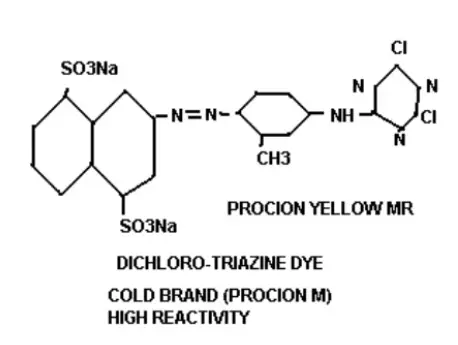
Bridging Group: These groups keep connections between the terminal reactive portion and chromophores. At figure (-NH-) act as a bridging group.
Solubilizing Groups: These groups help to solubilize. Dyeing is practiced in wet conditions. Materials are impregnated in water and Dyes and auxiliaries are injected and dozed in liquor. As these chemicals have high solubility, then they (chemicals) will easily come into contact and react (adsorption, adsorption, sorption, fixation) easily. Solubilizing groups are mainly polar groups and easily interact with water for dissolving. Example: In figure (-SO3Na) act as a solubilizing group.
Reactive Groups: These are the most important groups which represent the main mechanism of Dyeing. When dyes come into contact with the substrate the leaving group leave from the dye and dyes become positively charged. According to the reaction condition, a negative charge creates on the substrate (fiber/fabric). Leaving group (mostly Halogen) reacts with opposite charges that come from the substrate and form new compounds. Dyes connect with substrate and form covalent bonds. This covalent bond is fair and strong to meet wash and light fastness. According to the classification, reactive groups and leaving groups changed. In figure (-Cl) acts as leaving group.
- Author: Najmul Hossain
- Lecturer, Department of Textile Engineering
- Chittagong National Engineering College, Affiliated with University of Chittagong
- Email: [email protected] LinkedIn: Najmul Hossain
You may love to read: How to do Shade variation Control in Garments Industry
