Direct dyes are water-soluble anionic dyes and retain sulphonic acid groups in their structure. They are directly taken up by the fibers. In 1883, J. Walter of Geigy first discovered the synthetic direct dye, which was Direct Yellow 11. In 1956, SDC defined direct dye as, “Anionic dyes substantive to cellulose when applied from an aqueous bath containing electrolytes.” This article is about the Properties, Types, Chemical constitution, and Mechanism of Direct Dyes.
Dyes are retained on the fiber by hydrogen bonds and van der Waals forces. Though the light fastness of Direct dye is good but the wash fastness suffers a lot. As the dye particles are small and water-soluble, they exhibit poor wet fastness. However, it can be improved by after-treatments.
These dyes are superior to others in terms of cost, better light fastness, ease of application, shorter dye cycle, low cost of auxiliaries, remarkably lesser use of water, and much lower salt level in effluent.
Why are direct dyes called substantive dyes?
Direct dyes are also called Substantive dyes. These dyes have direct affinity to the fibers and adhere to the fibers by non-ionic forces. The dyeing process is cheaper. Dyes split up in water, forming the dye anion and the sodium cation.
Cellulosic materials, paper, leather, and so on are extensively dyed with direct dye. As direct dye particles are small and soluble in water, so, articles that are seldom washed, like window cover, bedding, upholstery, or products that are labeled with “Dry Clean Only” are often dyed with direct dye.
Properties of direct dyes
- Anionic in nature
- Good light fastness
- Poor wash fastness because of smaller particles with good water solubility, which can be improved by further after-treatment
- Mainly applied to cellulosic fibers, but suitable for protein fibers also, like nylon, silk, and wool
- Shorter dying cycle
- Ease of application
Types of Direct Dyes
Direct dyes are classified based on-
- Chemical structure
- SDC classification based on dyes’ levelling ability and their response to an increase in the dyeing temperature and to added salt during exhaust dyeing.
- Chemical structure: Most of the dyes belong to the azo dye class- Monoazo dyes (Diamine scarlet); Diazo dyes (Congo red); Triazo dyes (Direct brown); Polyazo dyes (Chlorazol dyes). A few are stibene derivatives
- SDC classification is as follows-
- SDC Class A direct dyes- SDC Class A dyes are self-leveling dyes. Even without the presence of salt and heat, these dyes have a good migration power. These dyes have lower substantivity. That’s why they need a proper amount of salt if we want a good exhaustion.
- SDC Class B direct dyes-SDC Class B dyes are salt-sensitive or salt-controllable dyes. These dyes have poor levelling characteristics. But if electrolyte is added in a controlled manner, even without any heat at the time of exhaustion, level dyeing is possible.
- SDC Class C direct dyes- SDC Class C dyes are very salt-sensitive dyes. They exhibit poor migration. Only the addition of salt is not effective for this dye. For a proper exhaustion, a considerable amount of salt is needed along with a proper additional temperature.
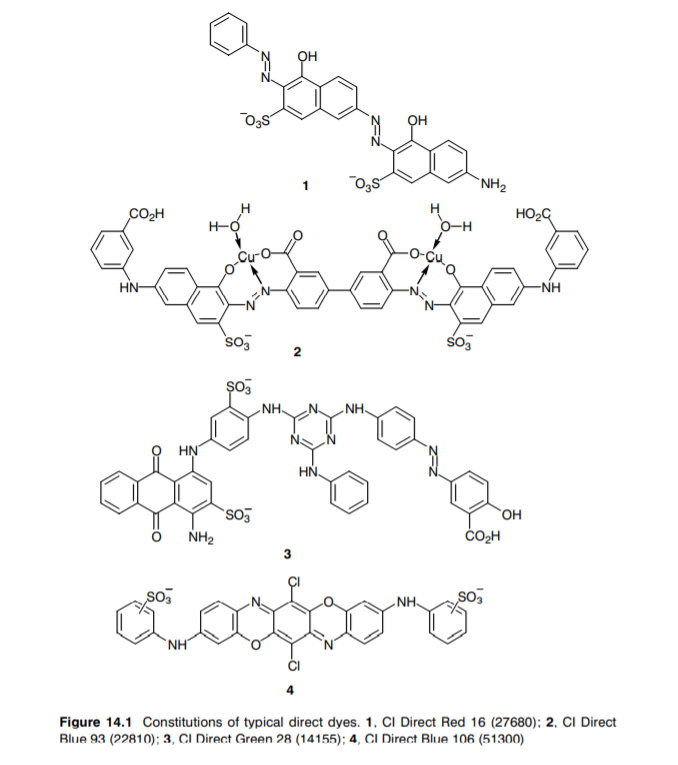
Chemical constitution of a direct dye
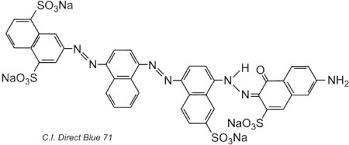
Sulphonated azo dyes constitute the predominant group of direct dyes. These are usually bis, tris-, or tetra-azo compounds, the tetra-azo type often being brown and black.
Mechanism of Direct Dyes
Mechanism of Direct Dyes: On immersion in water, the amorphous region of cellulosic fiber swells to produce small pores in the order of 20-100 A (Angstrom) and acquires negative charges.
Dye molecules also split in the bath and release dye anions. Initially, a few dye anions are absorbed on the surface of the fiber, but most of them are repelled from the surface. This occurs due to a huge negative potential difference, which is called the zeta potential barrier.
The addition of electrolytes reduces the zeta potential barrier and promotes absorption. Electrolytes release sodium cations that get attached to the dye molecules and carry them to the surface.
It also reduces the extent of osmotic work required to transfer the accompanying metal ions. The sodium salt of the dye molecule then deposits onto the fiber surface.
They gradually diffuse inside the swollen cellulose matrix. It places itself alongside the polymer chain with H-bond and van der Waals forces. Heat application helps to break dye aggregates and reduce the zeta potential barrier.
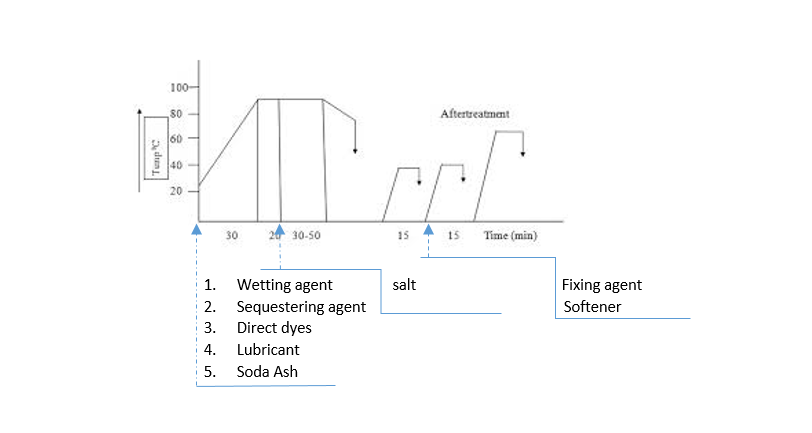
Direct Dyeing Process
- Typical recipe-
Wetting agent- 1-2 g/L
Sequestering agent- 1-2 g/L
Levelling agent- 0.5-1 g/L
Direct dyes- X%
Soda Ash- 1-5 g/L
Glauber/Common salt- 5-20 g/L or more (<0.5% shade= 5g/L; 1-2% shade= 10-20 g/L; 2-4% shade= 20-30 g/L; >4% shade= 30-40 g/L)
Temperature- 95-100℃
Time- 30-50 min
pH- Neutral to alkaline
M:L- 1:10
- Preparation of dye solution:
First, mix the dye with normal water and make a dye paste. Then, pour hot water to dissolve the dye properly and ensure bath concentration.
- Dyeing Procedure:
- Set the dye bath with substrate at room temperature
- Add dye solution with other auxiliaries and raise the temperature to 90℃
- Run the bath for 15-20 minutes and add salt gradually according to the depth of shade; the deeper the depth of shade, need more salt concentration
- It would be better if salt is added to the bath after reaching the temperature to boiling point. Maximum penetration is achieved during this period.
- Run the dye bath for 30-50 min at 90-95℃ to complete the cycle.
- Cool down the bath temperature to 60-70℃
- Drop the bath and rinse
- Then carry on the after-treatment process to improve wash fastness.
- After-treatment:
In the after-treatment process, a suitable fixing agent is used for improving wash fastness properties, which is generally done at 30-40℃, sometimes at 60℃ for 15-20 min or according to vendor recommendation. After that, a cationic softener is added to the last rinsing bath for improving the handle of the fabric. This process is done at 40-50℃ for 15-20 min.
The following recipe can be followed for the improvement of the (wash & light) fastness:
Bicromate or Fitcary – 0.5-2%
Copper sulfate – 0.5-2%
Acetic acid – 1-5%
Temperature- 80℃
Time – 30 min
M:L – 1:10
Dyeing Process Curve
Color Fastness Properties of Direct Dye
- Wash fastness: Poor due to small dye particles and having extreme water solubility
- Rubbing fastness: Moderate to Good
- Light fastness: Good
Factors influencing dye uptake
- Temperature
- pH
- Time
- Liquor ratio
- Affinity of dye
- Role of electrolyte
After-treatment of Direct dyes to improve wash fastness
The shades Direct dyes produce on cellulose are not wash fast as the dyes are water soluble and the molecular size of the dye is smaller than the pore size of cellulose. Wash fatness can be improved by increasing the molecular size of the dye through its reaction with other chemicals or dyes.
Various post-treatment methods are as follows:
- Treatment with a metal salt
- Treatment with formaldehyde
- Diazotization and development
- Coupling with diazotized base
- Topping with Basic dye
- Treatment with resin
Wrapping It Up!
Direct dye is the dye class that is more convenient to use. Costing, brilliancy, ease of use, everything makes it popular. But the one sacrificing thing is its wet fastness, but it can also be rectified through a chemical process. Substances that are seldom washed, like curtains, are highly dyed with direct dye. Properties, Types, Chemical Constitution, Mechanism of Direct Dyes
- You may love to read: Basic Anatomy of Reactive dye
- All the Trade Names of Reactive Dyes In Brief
- Difference Between Reactive and Vat Dyes
- Properties and Classification of Reactive Dyes
- Reactive Dyeing for Cotton Fabrics, Mechanism and Major Control Parameters
- Process Flow Chart of Viscose Fabric Dyeing
- Functions and Effect of Dispersing Agent in Dyeing Process
- Properties, Classification, Characters, and Mechanism of Acid Dye


